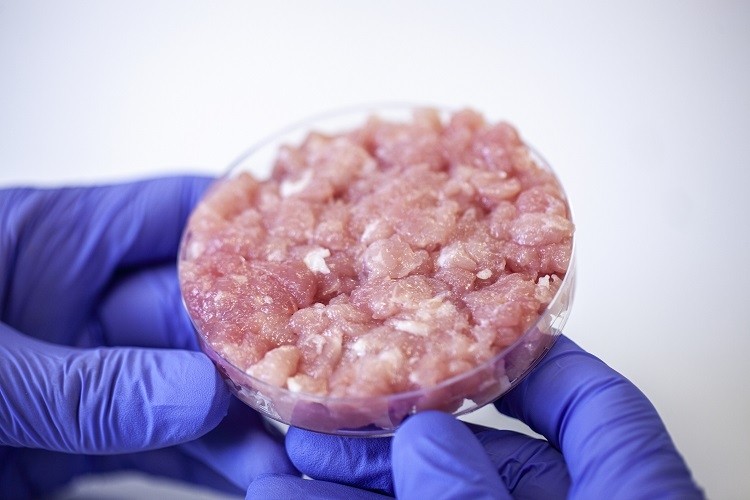
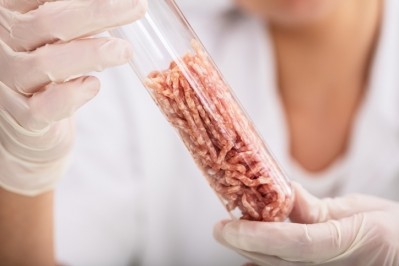
‘The race is on’: Is Japan’s commitment to cultivated meat another sign Europe is lagging?

Japan’s Prime Minister Fumio Kishida has announced plans to develop a cultivated meat industry.
“We will develop an environment to create a new market, such as efforts to ensure safety and development of labelling rules, and foster a food tech business originating in Japan,” he said, as reported by the Japan Economic Newspaper.
In Japan, no clear definition of cultured meat as food currently exists, nor have safety standards for raw materials and manufacturing processes been established. But the Government has shown willingness to advance this process.
“Food tech, including cellular foods, is an important technology from the perspective of realizing a sustainable food supply,” said Prime Minister Kishida. “We have to support efforts that contribute to solving the world’s food problems.”
The Good Food Institute (GFI) Europe has welcomed the move, describing it a ‘hugely significant step’ for cultivated meat globally. However, Acacia Smith, senior policy manager at GFI Europe suggested the EU must act to stay in the game.
“Japan now joins the rapidly growing list of countries recognizing the potential of sustainable proteins. The EU must use its new European Sovereignty Fund [for investment in European R&D and industrial projects] to invest in open-access research to create future-proof jobs and remain competitive in the global race to develop cultivated meat.”
‘Europe cannot rest on past accomplishments’
Other countries have similarly committed to developing a cultivated meat market. In late 2020, Singapore became the first country to approve cell-based meat for commercialization. Since then, the Singapore Food Agency (SFA) has also granted regulatory approval for the use of serum-free media in the production of cultivated meat.
The US is another country working to move cultivated meat products towards the market. Last year, Berkeley-based UPSIDE Foods received the greenlight from the Food and Drug Administration (FDA), marking the successful completion of its pre-market review.
And significant public investments have been made in China, Israel, and the Netherlands.
While the EU has a rich cultivated meat ecosystem – and indeed, is where cultivated meat was first created – any focus on developing a market appears hamstrung by stringent Novel Foods regulations: not one cultivated meat player has submitted a Novel Foods dossier to the European Food Safety Authority (EFSA).
Cellular Agriculture Europe, a coalition of food companies making slaughter-free meat, poultry, seafood and ingredients from animal cells, similarly welcomed Japan’s commitment. But acknowledged Europe ‘cannot rest on past accomplishments’.
“The members of Cellular Agriculture Europe welcome these developments in Japan, which come on the heels of the FDA greenlight in the US and massive public investments in cellular agriculture in China, Israel, and the Netherlands,” Robert E. Jones, president of Cellular Agriculture Europe, told FoodNavigator.
“Cultivated meat is a European innovation, we had the first regulatory guidelines, and the European Commission has granted millions of euros for research and development. However, we cannot rest on past accomplishments because the race is well underway to create the most attractive global hubs for this rapidly expanding industry.”
Are applicants put off the EU market due to bureaucracy?
There are signs that Singapore and the US – the two most advanced countries in terms of cultivated meat regulatory approvals – will continue to progress even further ahead.
According to IDTechEx, the first barrier to disrupting conventional meat production with cellular alternatives is regulation. While to date, Eat Just’s product in Singapore is the only commercially available cultured meat product globally, the UK-based consultancy says this will soon change.
“Several other leading players are also looking to enter the Singapore market. [And] on the other side of the world, regulatory approval from the US FDA for UPSIDE’s meat product indicates that the US market is opening soon.”
In contrast, the EU Parliament only had its first-ever debate on the topic of cultivated meat in 2022, noted IDTechEX, an indicator that the approval process may still be a while away.

EFSA is obviously aware it has yet to receive a Novel Foods dossier for a cultivated meat product but told us it is in ‘constant’ dialogue with stakeholders to ensure the regulatory requirements are clear.
The agency has also announced a colloquium in May to discuss requirements with stakeholders and explain the assessment process.
At the same time, EFSA has received an increased in applications for other novel foods, which it takes to mean applicants are not put off the EU market due to bureaucracy.
“In general, we have seen a steep rise in applications for new novel foods in Europe over recent years, which shows it’s clear that there is substantial interest and incentives in the EU market for such products,” an EFSA spokesperson told this publication.
There is more bureaucracy associated with getting novel foods to market in the EU than in other geographies however, because of its Member State structure. When a novel foods dossier is submitted, EFSA has a fixed deadline of nine months in which to complete its risk assessments. The clock may be stopped during this time if further data or clarifications are required.
According to the Novel Food Regulation, the European Commission has then seven months to prepare a proposal for a decision which is then made by a committee composed by EU Member State authorities (the so-called PAFF committee).
When the PAFF Committee casts its vote, a 55% majority ensures its approval. But, that 55% majority must represent 65% of the population of the EU.
“That decision and the timing of it is beyond EFSA’s remit,” the EFSA spokesperson explained.