GUEST ARTICLE
Industry Insights from NIZO - Polyphenols: Handling uninvited co-passengers in your plant protein

What causes differences in plant protein ingredients, even when they are from the same type of source? For instance, a protein isolate from one manufacturer may be darker, have a stronger taste, or act differently in a product than the ‘same’ protein isolate from another manufacturer.
While processing certainly plays a role, so does composition: the presence of ‘other’, possibly unwanted molecules in the protein isolate. One class of such ‘co-passengers’ is polyphenols.
Why should protein and food producers be paying close attention to polyphenols? And how can advances in their characterisation improve certain products? Renske Janssen, Project Manager Protein Technology for NIZO, explains.
René Floris: What are polyphenols, and where do they come from?
Renske Janssen: Polyphenols are compounds found in all plants. There are thousands of different kinds, such as flavonoids, phenolic acids and lignans. They can impact plant food's functionality in many ways, including flavour, colour, texture, mouthfeel, and more. For example, some polyphenols are associated with a bitter taste or astringent mouthfeel – think chocolate, tea, coffee, wine. But they can also have other flavour notes, such as ‘beany’, ‘earthy’ or even ‘dairy’. Furthermore, some may offer health benefits, for example as antioxidants.
RF: Why can they be a problem in plant proteins?
RJ: When you isolate protein from a plant source, around 80% of the isolate is the pure protein, but the other 20% is what we call ‘co-passengers’: other molecules, including polyphenols, carbohydrates, vitamins, enzymes, etc. In terms of concentration, polyphenols aren’t the most prevalent co-passenger, but even in small amounts, they can have a big impact. For example, pea proteins from different manufacturers can have very different yellow/brown tones, and different off-flavours, depending on which polyphenols are present. What’s more, the polyphenols can react, for example with oxygen in the product or atmosphere. This can potentially create stability issues and reduce shelf life by causing unwanted colours or flavours to develop over time, after the product has been produced.
RF: How do we reduce the negative impact of polyphenols?
RJ: Firstly, you have to know that polyphenols are in fact the cause of the problem you want to solve. Dark colour, for example, can be caused by polyphenols, but also by a Maillard reaction. You can then try to identify which polyphenols are causing the colour and where in the process they can be removed. Even small changes can have a big impact. But it’s a complicated process. To start with, you can have many different polyphenols in your protein isolate, not only depending on the type of plant source (i.e., pea, bean, almond), but also its genetic make-up (cultivar), the growing conditions and more. In addition, the behaviour of the polyphenols is very complex, especially when they bind with the protein. Sometimes, classifying the polyphenols to determine which ones are present and how they are interacting with the protein can provide useful insight.
RF: How do polyphenols interact with proteins?
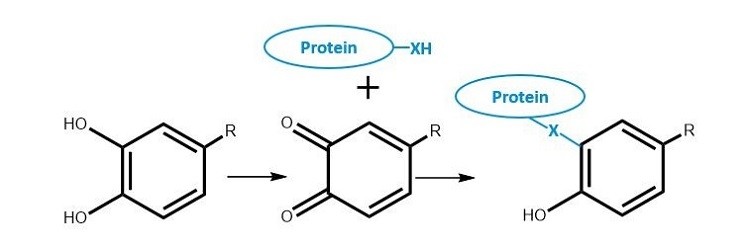
RJ: In some cases, the polyphenols remain completely separate from the protein. In others, polyphenols and proteins can form loose aggregates through hydrophobic interactions – think of how a mixture of oil and water separates. However, more reactive polyphenols can react with the protein to create new molecules through covalent bonding. To make matters more thorny, certain polyphenols in certain conditions can oxidise to create more reactive species, increasing the likelihood that they will bind covalently to the protein. Determining the type of bonding between the polyphenol and the protein can help you take targeted steps to solve the problem.
RF: What techniques can be used to characterise a polyphenol and its interaction with a protein?
RJ: We have taken big steps in identifying and characterising polyphenols in the past years at NIZO. We can combine the increased understanding of polyphenols with techniques such as mass spectrometry and high-performance liquid chromatography (HPLC) to understand what is going on in specific cases. For example, using chromatography, we can see difference in colour absorption of a protein, which indicates whether there is a polyphenol attached. We can then work with the customer to find strategies to get rid of the polyphenols. But there remains a lot to do, and the connection between proteins and polyphenols is still not always completely clear.
RF: How do you get rid of polyphenols in plant protein isolates?
RJ: If the polyphenol isn’t covalently bound to the protein, your first option is to eliminate as much as possible through washing, filtering (such as membrane filtration) and centrifuging. Covalently bound polyphenols are much more difficult to remove. One possibility is to adapt the processing conditions – including temperature, pH, salts - to inhibit oxidation and thereby reduce the chances of covalent bonding.
RF: What if you cannot eliminate the polyphenols?
RJ: Fermentation or bio purification may be used to modify the polyphenol and its behaviour. These techniques use living organisms, such as bacteria or yeasts, to convert the polyphenols through enzymatic conversion into more pleasant flavours. However, you have to know that the chosen microbes can convert your target polyphenol - at NIZO, for example, we have a large collection of strains for fermentation, enabling us to target specific molecules.
RF: How can the impact of the ‘good’ polyphenols be enhanced?
RJ: Polyphenols can also provide health benefits or give desired flavours to a product, like an almond flavour in an almond drink. In this case, you have to create a balance between eliminating off-flavours and retaining desired flavours. There’s no black and white solution, but greater understanding of the polyphenols can help put you on the right path.
In NIZO's next article, the research organisation will look at food safety in plant-based products.